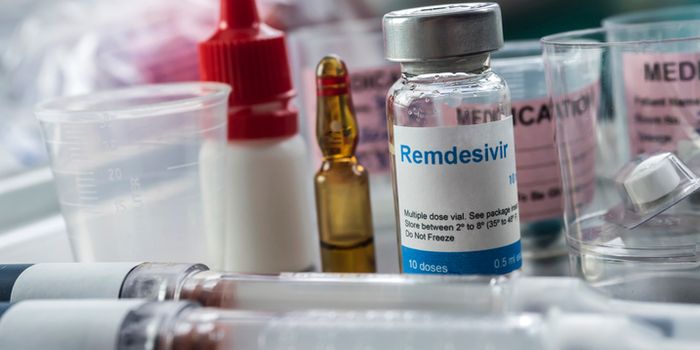
The antiviral drug remdesivir has been approved to treat Covid-19 patients over the age of 12 requiring hospitalisation.
The antiviral drug remdesivir has been named as the first drug to receive full approval to treat Covid-19 in the United States by the Food and Drug Administration (FDA).
In a statement, the FDA announced that approval had been granted for Veklury, the brand name of the remdesivir drug, for use in adult and paediatric patients aged 12 and older and weighing at least 40kg (just over six stone).
The FDA said that the drug was approved for treatment of Covid-19 that requires hospitalisation and that it should only be administered “in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care”.
The FDA had previously authorised remdesivir for emergency-use only in May.
The approval of Veklury was based on FDA analysis of data from three randomised, controlled clinical trials that included patients hospitalised with mild-to-severe cases of Covid-19.
According to the analysis, the time of recovery – defined as being discharged from the hospital or being hospitalised but not requiring supplemental oxygen and no longer requiring ongoing medical care – was cut by as much as five days in the group being treated with Veklury compared to a group being treated with a placebo.
The FDA also noted the potential side effects that arise as a result of using the drug, which include potential liver injury and allergic reactions, which may include changes in blood pressure and heart rate, low blood oxygen level, fever, shortness of breath, wheezing, swelling, rash, nausea, sweating or shivering.
The FDA approval comes after a study from the World Health Organisation (WHO) claimed last week that remdesivir had “little to no effect” on the survival prospects of Covid-19 patients.
Commenting on the FDA approval for the drug, FDA Commissioner Stephen M. Hahn, M.D. said: “The FDA is committed to expediting the development and availability of Covid-19 treatments during this unprecedented public health emergency.”
“Today’s approval is supported by data from multiple clinical trials that the agency has rigorously assessed and represents an important scientific milestone in the Covid-19 pandemic.
“As part of the FDA’s Coronavirus Treatment Acceleration Program, the agency will to continue to help move new medical products to patients as soon as possible, while at the same time determining whether they are effective and if their benefits outweigh their risks.”
LISTEN: You Must Be Jokin’ with Aideen McQueen – Faith healers, Coolock craic and Gigging as Gaeilge